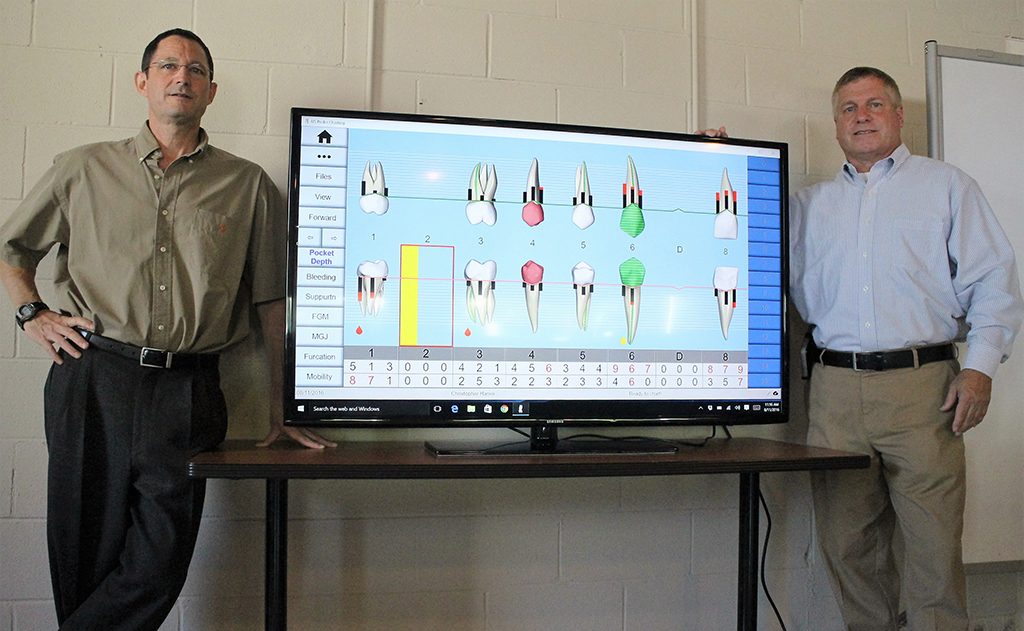
Todd Varon (left) and Jack Singer plan to role out charting software displayed on the monitor for its ultrasonographic probe in the next 30 days. (J. Elias O’Neal)
Jack Singer had spent more than 25 years running the numbers for a few high-profile firms in Richmond when he realized he’d had enough.
“I realized that I hated accounting,” said Singer, a New York native who came to Richmond to work for the likes of AMF Bowling and Best Products.
It was the early 1990s and Singer was working for Blue Cross and Blue Shield of Virginia.
“I wanted something for myself,” he said.
So he quit – and then the dentists came calling.
“I had worked with dentists and doctors during my time as comptroller at Blue Cross and Blue Shield,” Singer said. “I was doing some accounting work for a few dentists when they approached me about creating operatory software to consolidate charting and other dental records.”
From that point forward, Singer was hooked – eventually dropping his accounting accounts and making the switch to dental hardware development and sales.
Now, the entrepreneur is gearing up for his biggest pitch yet.
After years of development, Singer, along with business partner and co-founder Todd Varon, created U.S. Probe in 2014 – a company that’s nearing the launch of its main product: a handheld ultrasonographic probe that uses sound waves to help dentists identify potential pockets in a patient’s gums that could be prone to periodontal disease and other forms of decay.
Much of U.S. Probe’s product development has taken place at the Dominion Resources Innovation Center – a downtown Ashland-based facility that serves as an incubator for small tech businesses on the cusp of commercialization.
The probe’s handpiece component is undergoing design and clinical studies at VCU and Old Dominion University in Norfolk, Singer said.
Singer and Varon are working to secure regulatory approvals and patents in the U.S. by March 2017, Singer said. He added the firm is aiming to release its product into the market sometime during the fall of 2017, pending FDA approval.
Periodontal disease is caused by bacteria below the gum line, according to the National Institute of Dental and Craniofacial Research. If not treated, the gum disease can enter the blood stream and could contribute to low birth weight babies, cardiovascular disease, rapid tooth decay and strokes.
The company’s device uses a hollow tip that forces an acoustic beam into the gum tissue to identify the depth of the gum pocket.
Singer said the pocket depth is picked up through its charting software, where the dentist or hygienist can evaluate the depth, and whether the patient could be prone to infection or disease.
“If (the measurement) is a 1 or 2, then chances are things are OK,” Singer said. “But if it’s a 4, then it turns into a 4½ or a 6, then chances are that’s a good sign of the start of periodontal disease.”
Varon, who also worked at Blue Cross and Blue Shield until retiring in 2007, said the device is designed as a non-invasive, painless and more accurate alternative to other devices on the market that’s meant to serve as a preventive measure to combat disease and infection.
“Ultrasonic technology is very accurate,” Varon said. “The whole idea behind this is creating an accurate account of the patient’s dental health, and making sure that as the dentist inspects their gums they are comfortable.”
With the main handpiece component undergoing testing and development, the duo is preparing to roll out its accompanying software for the device, which includes dental charting software, in the next 30 days.
“The charting software is going to be the key to this product launch,” Singer said. “It’s going to help improve the accuracy.”
As the ultrasonographic handpiece launches its acoustic beam into the gums, U.S. Probe’s charting software will record, and read aloud, the depth level of the gums.
“Right now, the dentist takes the handpiece, digs into the gums and reads off a number aloud to the hygienist,” Singer said. “We realize that could lead to some mistakes during the procedure, so we wanted to develop software that would eliminate those errors.”
In its existing format, U.S. Probe’s charting system can be synced with other dental charting platforms that help identify tooth decay. The cloud-based software will be able to sync with most devices, including offices that use Apple, PC or Android devices, Singer added.
Singer said he and Varon each put up an undisclosed amount of personal capital to launch U.S. Probe.
The company has also received the backing of state and national organizations specializing in health and research innovation.
The Virginia Biosciences Health Research Corporation awarded U.S. Probe $536,000 for product development and research, and a $50,000 grant from the state’s Commonwealth Research Commercialization Fund in 2015.
U.S. Probe also received a $150,000 grant from the National Institute of Health’s Small Business Innovation Research program in 2005 to carry out additional work on the product line.
“The grants have helped us tremendously,” Singer said. “Much of the grant money is being used to complete the necessary clinical testing at VCU and ODU.”
And more grant awards are pending.
In October, Singer said U.S. Probe is set to receive another grant from the Commonwealth Research Commercialization Fund totaling $50,000. He added the company is in the process of submitting another grant application with the National Institutes of Health for $1.5 million.
“We feel that the revenue potential behind this product is going to be great,” Singer said.
U.S. Probe wants to deliver its device at a price that is competitive, although the firm is still researching what the device could sell for in the market.
Costs for existing dental utensils to help identify periodontal disease range from $25 to $4,500, Singer said.
With more than 150,000 registered dentists and 200,000 hygienists in the U.S., Singer said the market is ripe for this type of dental hardware.
“We feel it’s going to be a worthwhile tool,” Singer said. “Once we get it out to market, I think it’s going to appeal to a number of dentists.”
Then there’s revenue growth potential for dental firms.
By using U.S. Probe’s ultrasonographic device and accompanying software, Singer said dentists can pinpoint and immediately treat diseases that could worsen over time.
“You’re talking about identifying pockets of potential decay a lot quicker than with the current technology in the market, which could take months to identify,” Singer said. “With the device, dentists will be able to provide more advanced treatment…allowing them to provide more services and generate more income.
“It’s not just about our market share; it’s about what new services dental companies will be able to offer to their clients that will help boost their bottom line,” Singer said. “The impact is beyond just getting this product out to market.”

Todd Varon (left) and Jack Singer plan to role out charting software displayed on the monitor for its ultrasonographic probe in the next 30 days. (J. Elias O’Neal)
Jack Singer had spent more than 25 years running the numbers for a few high-profile firms in Richmond when he realized he’d had enough.
“I realized that I hated accounting,” said Singer, a New York native who came to Richmond to work for the likes of AMF Bowling and Best Products.
It was the early 1990s and Singer was working for Blue Cross and Blue Shield of Virginia.
“I wanted something for myself,” he said.
So he quit – and then the dentists came calling.
“I had worked with dentists and doctors during my time as comptroller at Blue Cross and Blue Shield,” Singer said. “I was doing some accounting work for a few dentists when they approached me about creating operatory software to consolidate charting and other dental records.”
From that point forward, Singer was hooked – eventually dropping his accounting accounts and making the switch to dental hardware development and sales.
Now, the entrepreneur is gearing up for his biggest pitch yet.
After years of development, Singer, along with business partner and co-founder Todd Varon, created U.S. Probe in 2014 – a company that’s nearing the launch of its main product: a handheld ultrasonographic probe that uses sound waves to help dentists identify potential pockets in a patient’s gums that could be prone to periodontal disease and other forms of decay.
Much of U.S. Probe’s product development has taken place at the Dominion Resources Innovation Center – a downtown Ashland-based facility that serves as an incubator for small tech businesses on the cusp of commercialization.
The probe’s handpiece component is undergoing design and clinical studies at VCU and Old Dominion University in Norfolk, Singer said.
Singer and Varon are working to secure regulatory approvals and patents in the U.S. by March 2017, Singer said. He added the firm is aiming to release its product into the market sometime during the fall of 2017, pending FDA approval.
Periodontal disease is caused by bacteria below the gum line, according to the National Institute of Dental and Craniofacial Research. If not treated, the gum disease can enter the blood stream and could contribute to low birth weight babies, cardiovascular disease, rapid tooth decay and strokes.
The company’s device uses a hollow tip that forces an acoustic beam into the gum tissue to identify the depth of the gum pocket.
Singer said the pocket depth is picked up through its charting software, where the dentist or hygienist can evaluate the depth, and whether the patient could be prone to infection or disease.
“If (the measurement) is a 1 or 2, then chances are things are OK,” Singer said. “But if it’s a 4, then it turns into a 4½ or a 6, then chances are that’s a good sign of the start of periodontal disease.”
Varon, who also worked at Blue Cross and Blue Shield until retiring in 2007, said the device is designed as a non-invasive, painless and more accurate alternative to other devices on the market that’s meant to serve as a preventive measure to combat disease and infection.
“Ultrasonic technology is very accurate,” Varon said. “The whole idea behind this is creating an accurate account of the patient’s dental health, and making sure that as the dentist inspects their gums they are comfortable.”
With the main handpiece component undergoing testing and development, the duo is preparing to roll out its accompanying software for the device, which includes dental charting software, in the next 30 days.
“The charting software is going to be the key to this product launch,” Singer said. “It’s going to help improve the accuracy.”
As the ultrasonographic handpiece launches its acoustic beam into the gums, U.S. Probe’s charting software will record, and read aloud, the depth level of the gums.
“Right now, the dentist takes the handpiece, digs into the gums and reads off a number aloud to the hygienist,” Singer said. “We realize that could lead to some mistakes during the procedure, so we wanted to develop software that would eliminate those errors.”
In its existing format, U.S. Probe’s charting system can be synced with other dental charting platforms that help identify tooth decay. The cloud-based software will be able to sync with most devices, including offices that use Apple, PC or Android devices, Singer added.
Singer said he and Varon each put up an undisclosed amount of personal capital to launch U.S. Probe.
The company has also received the backing of state and national organizations specializing in health and research innovation.
The Virginia Biosciences Health Research Corporation awarded U.S. Probe $536,000 for product development and research, and a $50,000 grant from the state’s Commonwealth Research Commercialization Fund in 2015.
U.S. Probe also received a $150,000 grant from the National Institute of Health’s Small Business Innovation Research program in 2005 to carry out additional work on the product line.
“The grants have helped us tremendously,” Singer said. “Much of the grant money is being used to complete the necessary clinical testing at VCU and ODU.”
And more grant awards are pending.
In October, Singer said U.S. Probe is set to receive another grant from the Commonwealth Research Commercialization Fund totaling $50,000. He added the company is in the process of submitting another grant application with the National Institutes of Health for $1.5 million.
“We feel that the revenue potential behind this product is going to be great,” Singer said.
U.S. Probe wants to deliver its device at a price that is competitive, although the firm is still researching what the device could sell for in the market.
Costs for existing dental utensils to help identify periodontal disease range from $25 to $4,500, Singer said.
With more than 150,000 registered dentists and 200,000 hygienists in the U.S., Singer said the market is ripe for this type of dental hardware.
“We feel it’s going to be a worthwhile tool,” Singer said. “Once we get it out to market, I think it’s going to appeal to a number of dentists.”
Then there’s revenue growth potential for dental firms.
By using U.S. Probe’s ultrasonographic device and accompanying software, Singer said dentists can pinpoint and immediately treat diseases that could worsen over time.
“You’re talking about identifying pockets of potential decay a lot quicker than with the current technology in the market, which could take months to identify,” Singer said. “With the device, dentists will be able to provide more advanced treatment…allowing them to provide more services and generate more income.
“It’s not just about our market share; it’s about what new services dental companies will be able to offer to their clients that will help boost their bottom line,” Singer said. “The impact is beyond just getting this product out to market.”


